First-In-Class Medicine
OncoSpherix’s lead compounds disrupt the activation of HIFs by preventing the recruitment of the transcriptional co-activator paralogs p300/CBP to the nascent transcription complex by binding to the CH-1 domain of p300/CBP.
Hypoxia-inducible factors (HIFs) are generally inactive in the presence of normal oxygen tension and active during hypoxia. This occurs because the alpha subunits undergo degradation in the presence of normal oxygen tensions and are stabilized under hypoxic conditions. The stabilization of the alpha subunits allows dimerization with the constitutively expressed beta subunit, and the dimer is then able to bind to specific elements on the DNA called hypoxic response elements (HREs). The HREs are found in the promoters of more than 100 genes that are regulated by HIFs. For transcription to occur, a co-activator protein is required: either p300 or CBP, proteins that are very similar in structure and function and hence are referred to as p300/CBP.
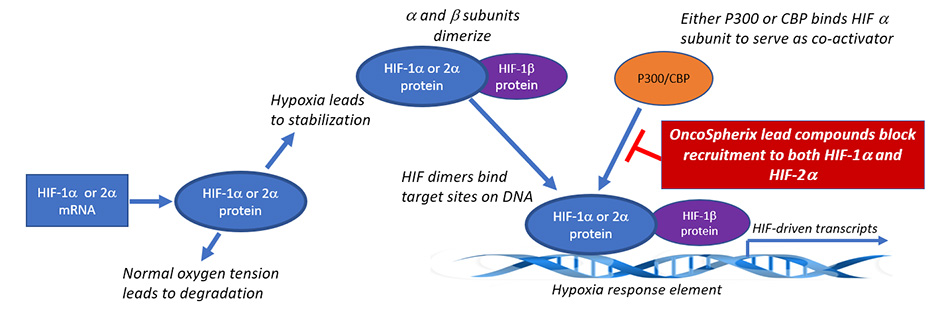
The α subunits of HIF-1 and HIF-2 are the oxygen sensitive subunits, while the β subunit (called HIF-1β for both HIF-1 and HIF-2) are constitutively expressed. In the presence of normal oxygen tension, the α subunits are rapidly degraded by the proteosome, whereas the a subunits are stabilized under hypoxic conditions and dimerize with the β subunit to initiate transcription factor complex formation. They bind to the hypoxia response elements in the DNA and recruit either P300 or CBP as a transcriptional cofactor. OncoSpherix’s lead family of compounds blocks the recruitment of P300/CBP, thereby preventing the expression of over 100 genes that are regulated by HIFs.
OncoSpherix’s lead family of compounds bind to p300/CBP on the CH1 domain, thereby disrupting the ability of p300/CBP to be recruited to the HIF transcriptional complex. This disruption occurs because structural elements in the CH1 domain of p300/CBP are needed for binding to both HIF-1α and HIF-2α.
Unlike the detrimental effects on normal cells that can result from complete inhibition of the function of p300/CBP, the binding of OncoSpherix compounds to p300/CBP is remote from its histone acetyltransferase enzymatic site. The binding of OncoSpherix also doesn’t affect the binding site for many other transcription factors. Thus, the transcription of p53 and of many other genes that use p300/CBP as a co-activator protein are not inhibited.
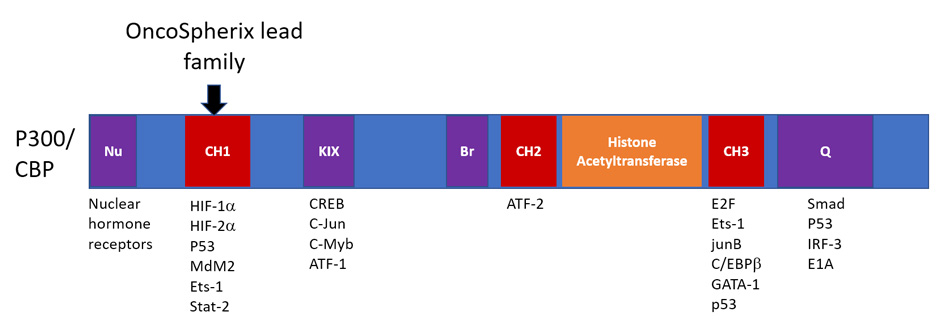
Schematic for P300/CBP: P300 and CBP are structurally related proteins that serve as cofactors for multiple transcription factors. The OncoSpherix lead family of HIF-inhibitors bind to the CH1 domain at a site that disrupts the binding of HIF-1α, HIF-2α while leaving intact the histone acetyltransferase site and the sites that are responsible for binding many other transcription factors.
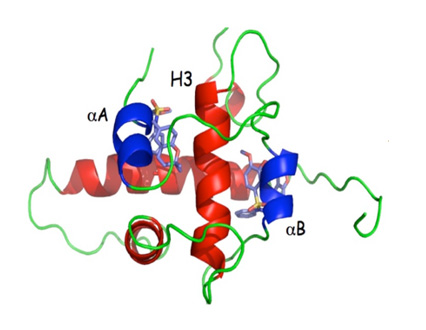
Two putative binding sites for OncoSpherix’ lead family of compounds flank helix H3 within the p300 CH-1 domain (red) at a site that interferes with binding to the HIF-α subunits. HIF-1α αA and αB helices are shown in blue (blue).
