Mission Driven Science
There has been tremendous progress in the fight against cancer, but there is much more to do
The death rate from cancer has declined by over 30% since its peak incidence in 1991, and this translates into millions of saved lives. Despite this wonderful fact, we still have a long way to go. Over 600,000 people in the US and over 9.6 million people worldwide still die from cancer each year.
 Advanced disease is the challenge
Advanced disease is the challenge
All too often, cancer cells invade critical structures and/or spread to multiple areas throughout the body, thereby preventing their complete removal by surgery or radiation. Traditional chemotherapy continues to be used to attack many types of cancer, and many exciting systemic therapies have been developed based on our growing knowledge of tumor biology. These include targeted therapies that disrupt specific molecular pathways which are responsible for driving the survival and spread of some types of cancer, and immunotherapies that unleash the power of the immune system to help attack cancer cells.
Some patients with specific types of cancer respond to these systemic therapies with complete and long-term elimination of their cancers. Unfortunately, the majority of patients have incomplete responses where transient clinical improvements are followed by progression of their cancer as the tumors become resistant to treatment.
OncoSpherix’s small molecule therapeutics attack a process that is common in most advanced cancers
OncoSpherix is committed to improving the quality of life and survival in people whose cancers cannot be cured by the standard of care. Most tumors grow faster than their blood supplies, which leads to regions where tumor cells are starved of oxygen, a situation known as hypoxia. Rather than being killed by the hypoxia, tumor cells fight back through the expression of master regulatory proteins called hypoxia inducible factors. These HIFs in turn drive the expression of over 100 genes that help tumor cells survive and spread, including genes that bring in new blood vessels (angiogenesis), help tumor cells resist cell death, help tumor cells invade into new areas and aid in the spread to remote regions (metastasis). HIF-driven genes also contribute to resistance to many types of therapy, including cytotoxic chemotherapy, radiation, and immunotherapies.
OncoSpherix small molecule therapeutics block HIF-driven gene expression so that tumor cells can’t thrive and spread from regions of hypoxia.
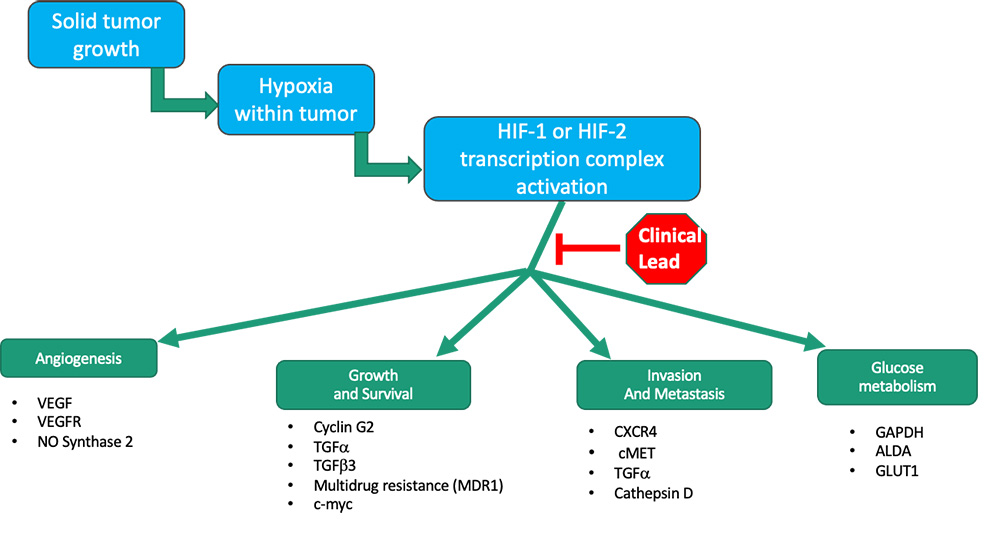
OncoSpherix’s lead family of compounds block the function of HIFs, thereby preventing this adaptive response so that tumor cells are more likely to die, and they are less likely to spread.
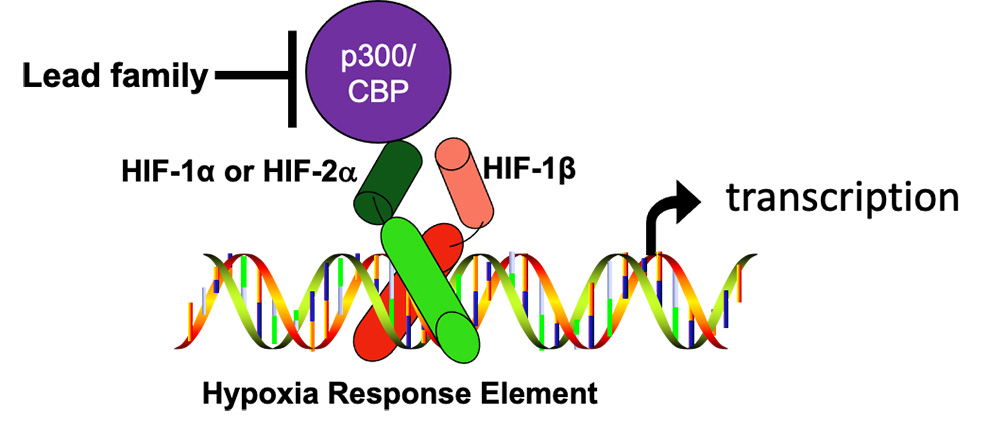
For a functional HIF complex to form, an alpha subunit (either HIF-1α, or HIF-2α) needs to join with a HIF-1β subunit, move into the nucleus, bind to specific targets in the DNA called hypoxia response elements (HREs), and recruit a transcriptional co-factor called p300/CBP. The formation of these functional complexes is blocked by OncoSpherix compounds that bind to a specific site on either p300 or CBP, transcription coactivator proteins that are structurally and functionally very similar (they are paralogs). This prevents p300/CBP from binding, especially to HIF-1α. As a consequence, transcription driven by HIF-1 is especially blocked.
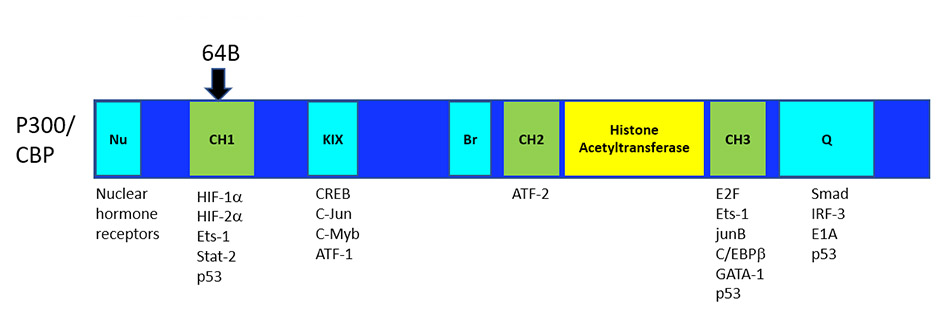
The site at which OncoSpherix’s lead family of compounds bind to p300/CBP leaves intact the ability of the P300/CBP cofactors to work with other important transcription factors. The binding site for the lead compounds is on the CH1 domain of p300/CBP at a site responsible for the interaction with both HIF-1α and HIF-2α, and hence causes the disruption of HIF-driven transcription. This binding site is remote from the histone acetyltransferase (HAT) enzymatic site and other important domains of p300/CBP, which probably accounts for the limited toxicity that has been observed, unlike the significant toxicity that is seen with agents that disrupt the HAT activity of p300/CBP.
The importance of the HIF pathway is reflected in the awarding of the Nobel Prize in Medicine and Physiology in October 2019 to three investigators “for their discoveries of how cells sense and adapt to oxygen availability.” The alpha subunits of HIFs are the ones that respond to changes in oxygen availability (HIF-1α and HIF-2α). When oxygen is abundant, the subunits are targeted for degradation so that a functional transcription complex cannot be formed. When oxygen is low, the alpha subunits are stabilized so that they are available to participate in transcription.
OncoSpherix’s lead family of compounds
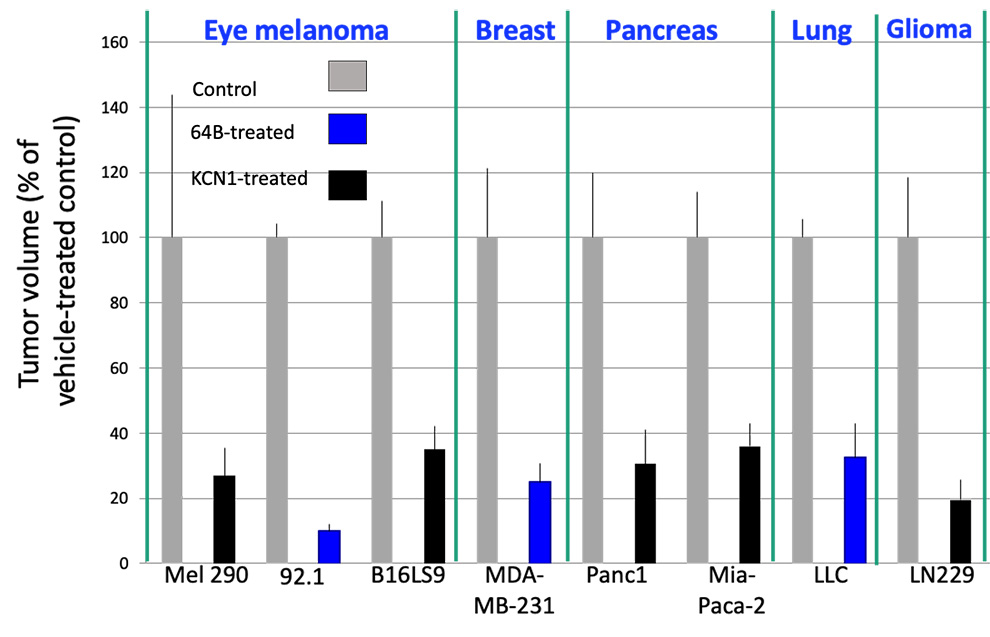
is effective in reducing primary tumor growth and metastasis in preclinical models of many types of cancer, including eye melanoma, breast cancer, pancreatic cancer, lung cancer and glioblastoma
Tumor cells were injected subcutaneously or orthotopically into mice, and two different compounds from the lead family (KCN1 and 64B) were delivered intraperitoneally daily. Final tumor volume (% of control) in vehicle and drug groups (10-12 mice/group) is shown.
Preclinical studies indicate that these compounds can be safely given with other systemic cancer treatments that work in well-oxygenated portions of tumors. The improved tumor control that occurs in response to these combinations is expected to improve the quality of life in patients with many types of advanced cancer. The efficacy of combining one of OncoSpherix’s dual HIF inhibitors with other systemic treatments, including targeted therapies and immunotherapies, is currently under evaluation, with the goal of combining treatments that work synergistically in the most effective and least toxic ways.
OncoSpherix’s focus is guided by science and motivated by medical need.
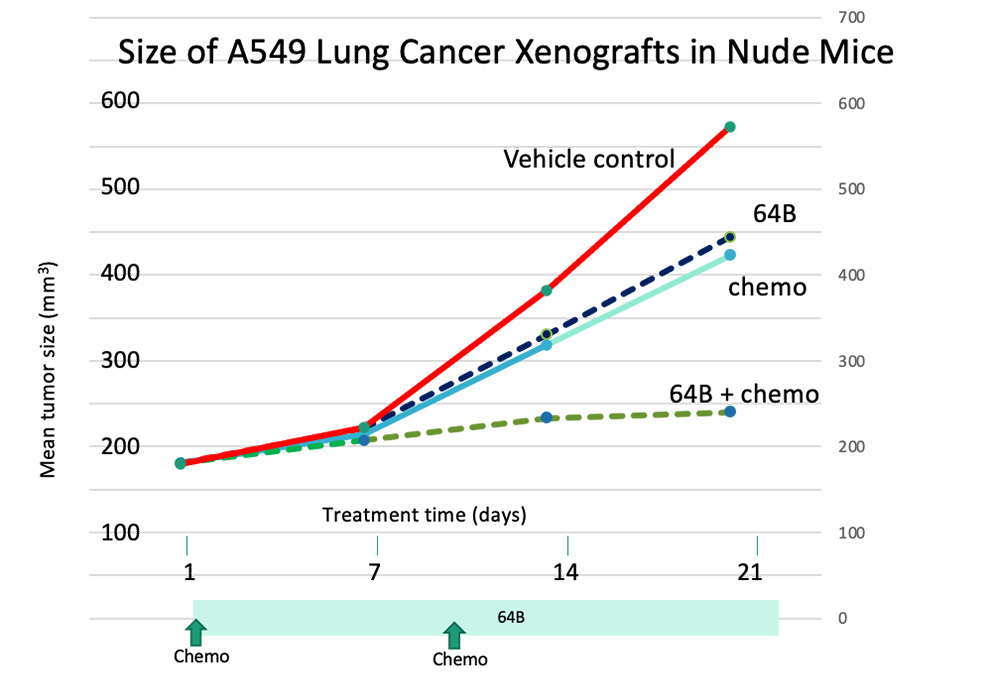
64B combined with cytotoxic chemotherapy synergistically inhibits growth of A549 lung adenocarcinoma xenografts in mice.
Treatment was initiated 2 weeks after implantation when tumors in each group of 10 mice ranged from 100-180 mm3 in size. Tumor measurements were done weekly. Each point shown is the average of 10 mice.